The European Union Medical Device Regulation, or EU MDR 2017/745, is a good package of regulations that are aimed at ensuring that medical devices in Europe are safe, operate properly, and are of good quality. Stiffer controls and harmonised new standards make manufacturers more responsible. Over 70 per cent of the manufacturers in the EU are incurring higher costs to comply with the stringent requirements of EU MDR 2017/745 compliance, indicating how complicated it is and how seriously the industry takes the issue of patient safety and fair markets.
Recent additions of the European Commission now require manufacturers should adhere to the new standards, including EN13795-1:2025 of surgical drapes and gowns and EN14683:2025 of medical face masks. These rules began on 20 October 2025. In order to meet the new standards, manufacturers should change their technical documents, conduct new tests, and change their Declarations of Conformity. This blog provides a compliance checklist of the Medical Device Regulation 2017 745, with the key elements that manufacturers have to adhere to remain in compliance.
Understanding and Complying with EU MDR 2017/745
- EU MDR 2017 745 is a set of strict rules regarding medical device safety, clinical testing, post-market monitoring, and record maintenance in Europe. It replaced the old Medical Device Directive 93/42/EEC in order to ensure that the rules keep up with new technology and provide greater protection to patients.
- Over 70% of manufacturers believe compliance rules make it more difficult and expensive to develop products. The new standards cover Decision (EU) 2025/2078, which entered into force on 20 October 2025.
Make sure that your medical devices are in accordance with EU MDR 2017 /745 – cooperate with Qualysec Technologies for verified evaluations and secure certification without problems!
Compliance Checklist EU MDR 2017/745

Be Accurate
- Determine the risk classification of your device (Class I, IIa, IIb, or III) according to Annex 8 of EU MDR 2017/745.
- Mistake classification can lead to delays or rejections and can attract the attention of the regulators.
- Assessment of recent changes to classification requirements in Regulation EU 2017 745.
Authorized Representative Outside EU
- Non-EU manufacturers must appoint a European Authorized Representative.
- Ensure transparent communication, registration, and compliance management by means of this appointed entity to meet EU MDR requirements.
Perform Clinical Evaluation and Investigation
- Strong clinical evidence should be presented as per Annex XIV, with reference to the latest regulations for high-risk devices.
- Conduct clinical investigations and on-going Post-semantic Clinical Follow-up (PMCF) with a focus on real-world performance, safety information, and in the context of EU Medical device regulation 2017 745.
Implementing a Post Market Surveillance (PMS) System
- Generate a proactive PMS plan that includes vigilance, complaints, and Periodic Safety Update Reports (PSUR) for higher-class devices.
- Class I, IIa, IIb, and III devices must work with a Notified Body for Conformity assessment and Certification. Read our Cybersecurity Assessment
- Revise technical documents, validation records, and verification reports based on the harmonised standards which have been in force since 20 October 2025.
Receive Notified Body CE Certification
- Classify your device according to Annex 8 of EU MDR 2017/745.
- A false classification can lead to delays, refusals, or additional inspection by the regulatory authorities.
- Re-examining for new changes of classification rules under Regulation EU2017/745.
Registration of Devices and Economic Operators in EUDAMED
- Add your devices, manufacturers, authorised reps, and importers to the European Database on Medical Devices (EUDAMED) as mandatory by Regulation EU 2017 745.
- Keep information updated.
Update Products Labelling & UDI Requirements
- Product labelling and packaging fully meet Annex I Section 23 requirements and include Unique Device Identification requirements.
- Revise Declarations of Conformity and Instructions for Use to refer to the revised harmonized standards introduced by the latest EU implementing decision.
Keep Risk Management and GSPR in Compliance
- Follow a lifecycle risk management process, with hazard identification, benefit-risk analysis, and regular updates; and
- Demonstrate Conformity to General Safety and Performance Requirements (GSPRs) in aspects of both the design of the product and the biocompatibility, usability, and labelling of the product.
Supplier and Material Compliance Documentation
- Verify supplier compliance with newly revised requirements for all raw materials and components, particularly fabrics, filtration, and sterilisation systems, while ensuring healthcare security compliance.
- Absolute non-conformance to suppliers – update contracts and control agreements to stop downstream non-conformance.
Plan forlude and Inspections
- Always be prepared for an audit by EU authorities and Notified Bodies with documents, labels, and records.
- Watch local compliance as amendments and changes to the EU Medical Device Regulation 2017 745.
Follow Transitional Deadlines and Regulations
- Adopt recently harmonized standards as soon as possible, which is required by Decision(EU)2025/2078 – no formal transition period is applicable.
- Take advantage of delayed compliance deadlines during phased transitional periods, having final compliance deadlines for most device categories until May 2029. Learn about Medical IoT Security.
Keep track of National and European Amendments
- Follow regularly updated of local health authorities and the European Commission, such as Guidance Documents for the Management of COVID-19 and the mitigation of occupational risks by the Medical Devices Coordination Group (MDCG) in Germany (BfArM).
- Monitoring changes related to conformity, technical documents, and access to the market.
Download the Sample Penetration Testing Report – see how Qualysec ensures EU MDR 2017/745 compliance with secure, tested medical devices.
Download the Exclusive Pen Testing Report

How Qualysec Technologies Can Help You
By adhering to the items in this checklist, medical device organisations can greatly minimise compliance risks, enhance product safety, and secure smooth market access under EUMDR 2017 745 and general EU 2017 745 regulatory environments.
Qualysec Technologies is the partner of choice for companies requiring assistance with the EU MDR 2017/745 compliance and medical device penetration testing. The easy-to-follow step-by-step test procedure offers precise and verified test results for European medical devices.
How Qualysec supports your regulatory journey
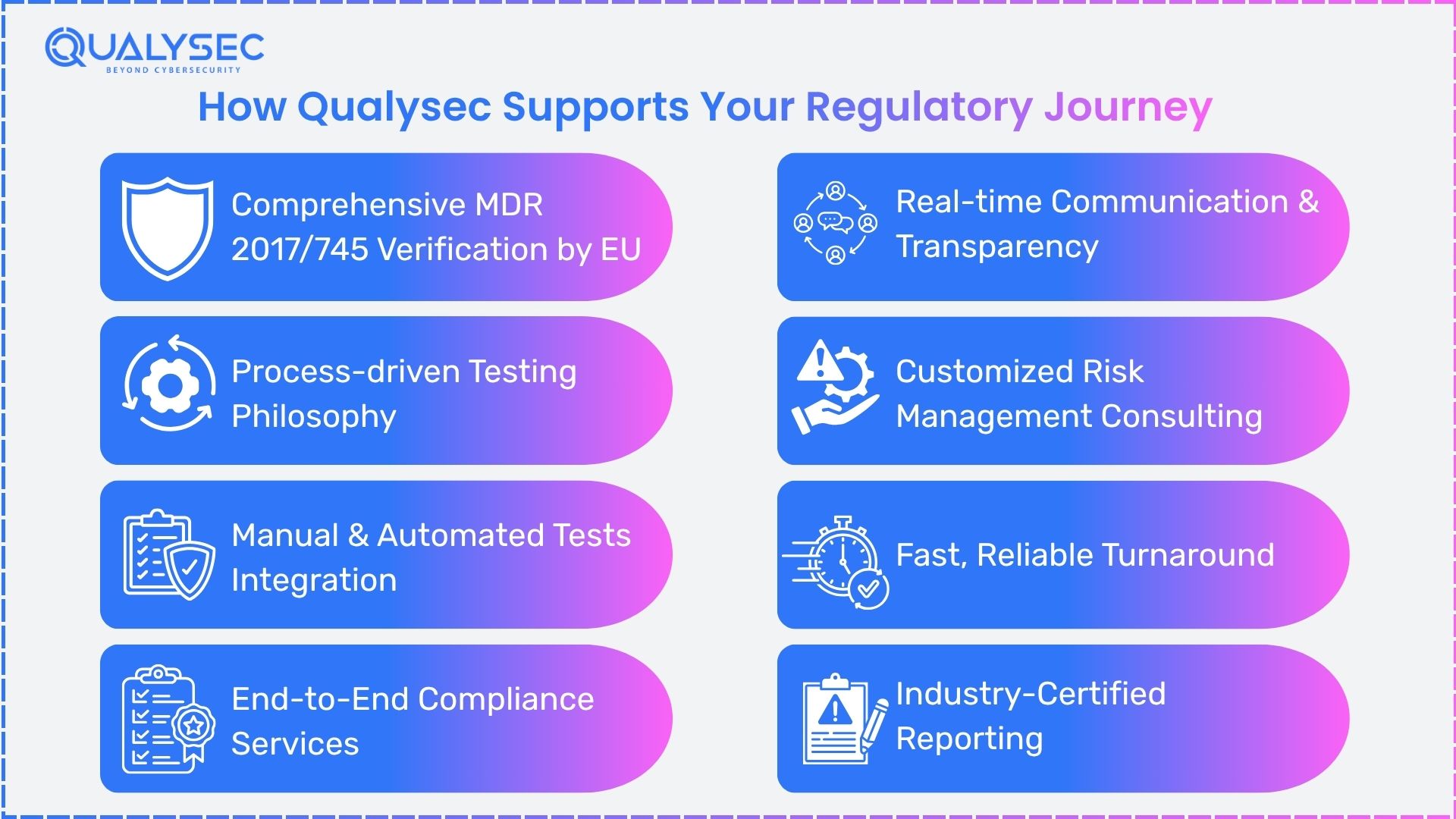
- Comprehensive MDR 2017/745 Verification by EU –
We ensure that every project is produced to the latest harmonized standards and the specific requirements of EU MDR 2017/745, so that you are prepared for audits and certifications.
- Process-driven Testing Philosophy –
We have a unique, process-based approach to testing that checks your clinical, technical, and post-market compliance. This helps us discover and correct gaps in documentation, risk management, and clinical evidence, which are directly related to the clauses of the regulation.
- Manual and Automated Tests Integration –
Every interaction is supported by expert inspectors and automated systems to identify technical, procedural, and data errors. This combined way decreases false alarms and generates a transparent and clear compliance audit report.
- End-to-End Compliance Services –
Our services include technical file review, UDI readiness, QMS review, post-market surveillance, clinical evaluation checks, and support for Notified Body interviews. We train your people to confidently respond to any review.
- Real Time Communication and Transparency –
Clients are engaged with us in a partnership manner, receiving frequent updates, clear results, and education throughout the compliance process.
- Customized Risk Management Consulting –
We customize solutions to your device type, company size, and regulatory history. Our experts carry out risk-based security testing, tailored to the risk profile of your product, expediting your product to market.
- Fast, Reliable Turnaround –
We accelerate the certification and introduction to the market thanks to a quick and well-structured process, ensuring the quality is maintained and in line with changing European rules concerning medical devices.
- Industry‑Certified Reporting –
Every report complies with EU MDR 2017/745 and associated regulation, providing you with the technical and procedural evidence for internal reviews and Notified Body checks.
Want to live the life of clear and confident compliance? Contact Qualysec Technologies today – your first step to smooth and reliable EU MDR 2017/745 fulfilment!
Conclusion
In summary, compliance with Medical Device Regulation 2017 745 is a must for any manufacturer that desires to sell in Europe. Keep a track of the changes in the rules, follow the checklist very carefully, and avail trustworthy healthcare device pentesting services like Qualysec Technologies.
This increases your chances of certification timeliness and a good sale. Don’t risk breaking the rules – get some expert help to get through the changing rules with confidence.
Speak directly with Qualysec’s certified professionals to identify vulnerabilities before attackers do.
FAQs
1. What is 745 in MDR?
745 in EU MDR 2017 745 regulation establishes safety, performance, and quality standards of medical devices sold in the EU. It replaced the old Medical Device Directive and has made patient safety and the consistency of the markets better.
2. What are the four main classifications of EU MDR 2017 745?
Devices are in four risk groups:
- Class I (low risk),
- IIa Class (medium risk for short-term usage),
- Class IIb (medium-high risk for long-term or implanted usage), and
- Class III (high risk).
The class decides on the amount of regulation and testing needed.
3. What are the new EU MDR requirements?
They include coverage of more devices, hear clinical evidence, increased use of strengthened Unique Device Identification (UDI) for tracking, increased post-marketing monitoring, and added transparency through UDAMED registration and stricter controls on suppliers. All are meant to make patients safer and devices reliable.
4. What is regulation 2017 745 and 2017 746?
Decree 2017/745 is about medical devices. The in vitro diagnostic tests (IVDR) are subject to Regulation 2017/746. Together, they establish the current EU rules with high requirements on safety, testing, and monitoring markets.
Start your EU MDR 2017/745 compliance today with Qualysec Technologies – Contact now!










































































































































































































































































































































































































































































































0 Comments